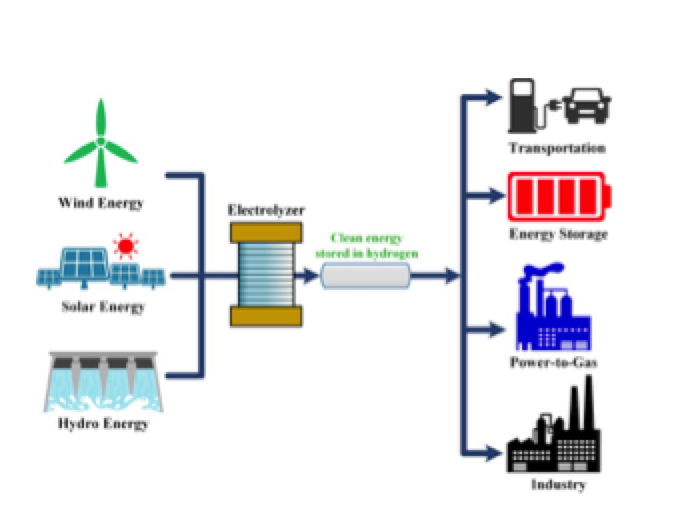
Hydrogen is the most abundant element in the universe. It appears, in its free state, at atmospheric pressure and ambient temperature, in the form of a diatomic gas with the formula H2. On Earth, however, it is scarcely present in the free and molecular state and must therefore be produced. There are many consolidated industrial uses in the chemical and petrochemical industry (refining of fossil fuels and in ammonia synthesis). More recently, the focus has been on the use of this gas as a clean automotive fuel and to produce electricity mitigating CO2 emissions.
Most of the hydrogen currently produced (about 96%) uses the steam-reforming process from methane gas (or ethanol or propane) with a high temperature reaction; however, at the end of the process CO2 is also obtained. Alternatively, it is possible to produce hydrogen from the water molecule by exploiting electricity through an electrolyzer. This process is inherently clean because only oxygen is obtained as a waste product. Of course, the way in which electricity is produced must be taken into account: if fossil fuels are used, CO2 generation is caused indirectly (the hydrogen thus obtained is called gray); for this reason the use of renewable sources (obtaining green hydrogen) would be preferred.
Once produced, hydrogen can be stored and reused when necessary. In this way it is proposed as an alternative to traditional batteries; in fuel cells, in fact, electricity is produced by converting the hydrogen with an inverse process to that of electrolysis.
It may seem unreasonable to produce hydrogen with electricity and then reuse it to produce electricity again. In reality this is already happening by battery from everyday objects such as telephones to electric vehicles. It therefore makes sense to consider an alternative energy carrier that does not involve CO2 emissions.
The use of renewable energy sources has posed new challenges in terms of research to make the conversion of electricity into hydrogen increasingly efficient. The problems to be solved are related to power electronic conversion circuits and the variability of climatic conditions. The former are necessary for the voltage produced by a renewable source to be continuous (photovoltaic system) or alternating (wind turbine) to be adapted to a suitable value to supply the electrolyzer. The variability of the source also requires appropriate models of electrolysers able to reproduce the dynamics.
At ICAR, in collaboration with the “GREEN” laboratory in Nancy, part of the Université de Lorraine (FR) and the University of Technology North Bangkok (KMUTNB), both power converters to supply the electrolyzers and dynamic models of electrolyzers to reproduce their functions in the laboratory are being studied. Recently, new circuit topologies have been proposed for PEM (proton exchange membrane) electrolyzer applications, which are available on the market with power ratings starting from one hundred watts and therefore would also be suitable for domestic use, and dynamic models of the same.
Sitography to learn more
https://www.energy.gov/eere/fuelcells/hydrogen-storage
https://www.cleantech.com/the-role-of-green-hydrogen-in-global-decarbonization/
https://www.hydrogen.energy.gov/annual_review19_report.html
https://www.frontier-economics.com/media/3120/value-of-gas-infrastructure-report.pdf
https://ieaghg.org/docs/General_Docs/Reports/Ph4-24%20Hydrogen%20in%20nat%20gas.pdf
https://www.iea.org/tcep/energyintegration/hydrogen/
https://www.mdpi.com/1996-1944/12/12/1973/htm
https://www.nrel.gov/docs/fy13osti/51995.pdf
https://www.hydrogen.energy.gov/pdfs/review19/2019_amr_report_hydrogen_fuel.pdf
http://www.icrepq.com/icrepq%2716/PL1.pdf
https://www.rees-journal.org/articles/rees/pdf/2016/01/rees160035-s.pdf
Last published papers
- Yodwong, B., Guilbert, D., Phattanasak, M., Kaewmanee, W., Hinaje, M., & Vitale, G. (2020). Proton Exchange Membrane Electrolyzer Modeling for Power Electronics Control: A Short Review. C—Journal of Carbon Research, 6(2), 29.
- Yodwong, B., Guilbert, D., Phattanasak, M., Kaewmanee, W., Hinaje, M., & Vitale, G. (2020). AC-DC Converters for Electrolyzer Applications: State of the Art and Future Challenges. Electronics, 9(6), 912.
- Guida, V., Guilbert, D., Vitale, G., & Douine, B. (2020). Design and Realization of a Stacked Interleaved DC–DC Step‐Down Converter for PEM Water Electrolysis with Improved Current Control. Fuel Cells.
- Alonge, F., Collura, S. M., D’ippolito, F., Guilbert, D., Luna, M., & Vitale, G. (2020). Design of a robust controller for DC/DC converter–electrolyzer systems supplied by μWECSs subject to highly fluctuating wind speed. Control Engineering Practice, 98, 104383.
- Guilbert, D., & Vitale, G. (2020). Improved Hydrogen-Production-Based Power Management Control of a Wind Turbine Conversion System Coupled with Multistack Proton Exchange Membrane Electrolyzers. Energies, 13(5), 1239.
- Guilbert, D., Sorbera, D., & Vitale, G. (2020). A stacked interleaved DC-DC buck converter for proton exchange membrane electrolyzer applications: Design and experimental validation. International Journal of Hydrogen Energy, 45(1), 64-79.
- Collura, S. M., Guilbert, D., Vitale, G., Luna, M., Alonge, F., d’Ippolito, F., & Scipioni, A. (2019). Design and experimental validation of a high voltage ratio DC/DC converter for proton exchange membrane electrolyzer applications. International Journal of Hydrogen Energy, 44(14), 7059-7072.
- Guilbert, D., & Vitale, G. (2019). Dynamic Emulation of a PEM Electrolyzer by Time Constant Based Exponential Model. Energies, 12(4), 750.